Patient-centred pharmacovigilance to improve reporting of Adverse Drug Reactions amongst those living with HIV and TB in Uganda
GCRF Funding Cycle
2019-20
Principal Investigator
Derek Sloan
Schools
Medicine
ODA countries
Uganda
Sustainable Development Goals
Goal 3
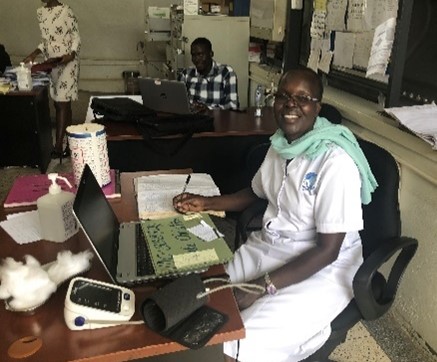
Tuberculosis and HIV are major public health problems in Uganda. People suffering from these illnesses sometimes find it difficult to fully adhere to prolonged medical treatments, some of which are complicated by drug-drug interactions and side-effects. Careful study of attitudes and behaviours in relation to treatment adherence and toxicity are lacking. In this project, we interviewed patients and healthcare workers from our clinical trial, which we are running to determine whether higher doses of the anti-TB drug rifampicin can be given safely to TB-HIV patients, to better understand and learn from their experiences. Adapting the project to accommodate the emerging Covid-19 pandemic, we also disseminated multi-media public health information broadcasts to combat “myths” and “misinformation” about virus transmission and treatment.
So far, we have recruited almost all of the 120 patients required for the clinical trial and shown that higher doses of the anti-TB drug, rifampicin, do not reduce the effectiveness of HIV treatment, which is important. We will present these results at a major international conference in 2021. We also have a detailed report of qualitative responses from patients and healthcare workers from Focus Group Discussions, which we are developing for publication and for presentation to public health authorities in Uganda. We have produced, and disseminated (using national TV/radio and social media) a series of public-facing broadcasts to transmit crucial, accurate scientific messages on Covid-19.